Calculate the molarity of the following aqueous solutions: (c) 25.0 mL of 3.50 M HNO3 diluted to 0.250 L.
Ascorbic acid (vitamin C, C6H8O6) is a water-soluble vitamin. A solution containing 80.5 g of ascorbic acid dissolved in 210 g of water has a density of 1.22 g/mL at 55 °C. Calculate (c) the molality,
 Verified step by step guidance
Verified step by step guidance
Verified Solution
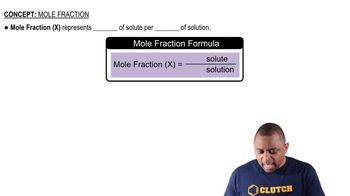
Key Concepts
Molality

Moles of Solute
Density and Volume Relationship

What is the molarity of each of the following solutions: (b) 5.25 g of Mn(NO3)2⋅2H2O in 175 mL of solution,
Ascorbic acid (vitamin C, C6H8O6) is a water-soluble vitamin. A solution containing 80.5 g of ascorbic acid dissolved in 210 g of water has a density of 1.22 g/mL at 55 °C. Calculate (a) the mass percentage,
The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (a) What is the mole fraction of methanol in the solution?
The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (b) What is the molality of the solution?
The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (c) Assuming that the volumes are additive, what is the molarity of CH3OH in the solution?
