A 35.1 g sample of solid CO2 (dry ice) is added to a container at a temperature of 100 K with a volume of 4.0 L. If the container is evacuated (all of the gas is removed), sealed, and then allowed to warm to room temperature (𝑇=298 K) so that all of the solid CO2 is converted to a gas, what is the pressure inside the container?
Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 895 torr and 24°C. c. At what temperature will the volume be 15.00 L if the pressure is 8.76×102 torr?

Verified Solution
Key Concepts
Ideal Gas Law

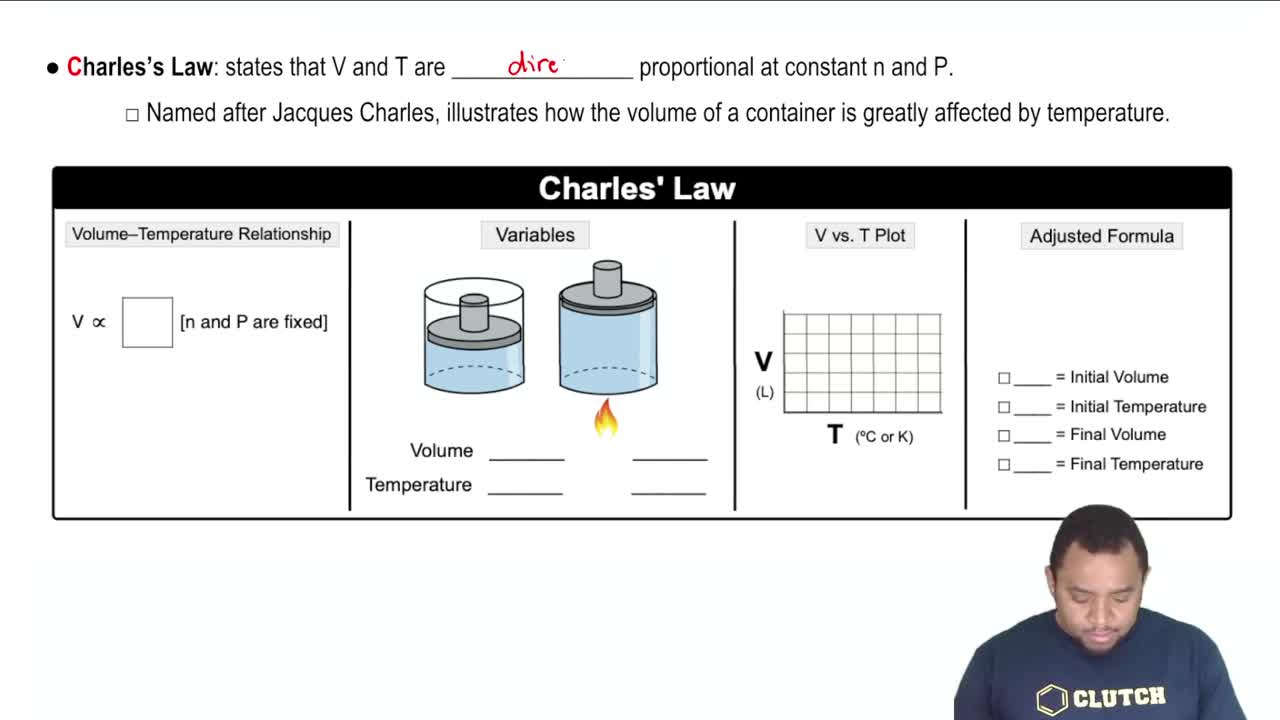
Charles's Law
Gas Pressure Units

A 334-mL cylinder for use in chemistry lectures contains 5.225 g of helium at 23°C. How many grams of helium must be released to reduce the pressure to 75 atm assuming ideal-gas behavior?
Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 895 torr and 24°C. b. What volume will the Cl2 occupy at STP?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 55.0 gallons that contains O2 gas at a pressure of 16,500 kPa at 23°C. b. What volume would the gas occupy at STP?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 55.0 gallons that contains O2 gas at a pressure of 16,500 kPa at 23°C. c. At what temperature would the pressure in the tank equal 150.0 atm?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 55.0 gallons that contains O2 gas at a pressure of 16,500 kPa at 23°C. d. What would be the pressure of the gas, in kPa, if it were transferred to a container at 24°C whose volume is 55.0 L?
