Indicate whether each statement is true or false. (c) Molecules containing electrons that occupy antibonding orbitals must be unstable.
How would we describe a substance that contains only paired electrons and is weakly repelled by a magnetic field? Which of the following ions would you expect to possess similar characteristics: H2-, Ne2+, F2, O22 +?

Verified Solution
Key Concepts
Paired Electrons

Diamagnetism
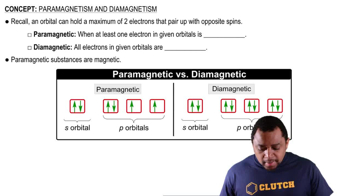
Ionic Characteristics

Indicate whether each statement is true or false. (d) Electrons cannot occupy a nonbonding orbital.
Using Figures 9.35 and 9.43 as guides, draw the molecular orbital electron configuration for (d) Ne22 +. In each case indicate whether the addition of an electron to the ion would increase or decrease the bond order of the species.
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of (b) NO+.
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of (d) ClF.
