NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (b) Use the ionic radii given in Figure 7.8 to estimate the Na─Cl and K─F distances.
Ch.8 - Basic Concepts of Chemical Bonding
Chapter 8, Problem 23d
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (d) Using the lattice energies in Table 8.1, predict the lattice energy of ScN.
 Verified step by step guidance
Verified step by step guidance1
Identify the concept of lattice energy, which is the energy required to separate one mole of an ionic solid into its gaseous ions.
Understand that lattice energy is influenced by the charges of the ions and the distance between them, as described by Coulomb's Law.
Recognize that ScN is composed of Sc^{3+} and N^{3-} ions, which have higher charges compared to NaF and CaO, leading to a higher lattice energy.
Use the lattice energies of NaF and CaO from Table 8.1 as reference points, noting that higher charges in ScN will result in a significantly higher lattice energy.
Predict that the lattice energy of ScN will be greater than those of NaF and CaO due to the higher ionic charges, without calculating the exact value.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectronic Species
Isoelectronic species are atoms, ions, or molecules that have the same number of electrons and, consequently, the same electronic structure. For example, NaF and CaO both have the same number of valence electrons, which influences their chemical properties and interactions. Understanding isoelectronic relationships helps predict the behavior of compounds in terms of stability and reactivity.
Recommended video:
Guided course
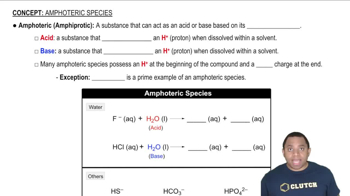
Amphoteric Species
Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form an ionic solid, or the energy required to separate one mole of a solid ionic compound into its gaseous ions. It is a measure of the strength of the forces between the ions in an ionic compound. Higher lattice energies indicate stronger ionic bonds, which can affect the stability and solubility of the compound.
Recommended video:
Guided course

Lattice Energy
Trends in Lattice Energy
Lattice energy trends can be predicted based on ionic charge and ionic size. Generally, greater charges on the ions lead to higher lattice energies due to stronger electrostatic attractions, while larger ionic radii result in lower lattice energies due to increased distance between the charged ions. Understanding these trends is essential for predicting the lattice energy of compounds like ScN based on known values from similar compounds.
Recommended video:
Guided course

Lattice Energy
Related Practice
Textbook Question
545
views
Textbook Question
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (a) What are the charges on each of the cations in each compound?
806
views
Textbook Question
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (b) What are the charges of each of the anions in each compound?
403
views
Textbook Question
(a) Does the lattice energy of an ionic solid increase or decrease (i) as the charges of the ions increase, (ii) as the sizes of the ions increase?
807
views
Textbook Question
Consider the ionic compounds KF, NaCl, NaBr, and LiCl. (a) Use ionic radii (Figure 7.8) to estimate the cation–anion distance for each compound.
1072
views
Textbook Question
Which of the following trends in lattice energy is due to differences in ionic radii? (a) LiF > NaF > CsF, (b) CaO > KCl, (c) PbS > Li2O.
764
views
