Here are the essential concepts you must grasp in order to answer the question correctly.
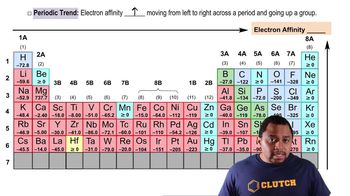
Electron Affinity
Electron affinity is the amount of energy released when an electron is added to a neutral atom in the gas phase to form a negative ion. A negative electron affinity indicates that energy is required to add an electron, meaning the process is endothermic. Understanding this concept is crucial for determining the stability of anions and the tendency of elements to gain electrons.
Recommended video:
Iodine and Iodide Ion
Iodine (I) is a neutral atom, while the iodide ion (I-) is the anion formed when iodine gains an electron. The electron affinity of iodine can be compared to that of the iodide ion to understand their respective tendencies to accept electrons. This comparison is essential for predicting the behavior of these species in chemical reactions.
Recommended video:
Trends in Electron Affinity
Electron affinity varies across the periodic table, generally increasing from left to right and decreasing from top to bottom. Halogens, like iodine, typically have high electron affinities due to their desire to achieve a stable electron configuration. Recognizing these trends helps in predicting which species will have a negative electron affinity based on their position in the periodic table.
Recommended video:
 Verified step by step guidance
Verified step by step guidance