Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic vs. Molecular Compounds
Ionic compounds are formed when electrons are transferred from one atom to another, typically between metals and nonmetals, resulting in charged ions that attract each other. In contrast, molecular compounds consist of atoms that share electrons through covalent bonds, usually between nonmetals. Understanding the nature of the bonding helps in predicting whether a compound is ionic or molecular.
Recommended video:
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. When the difference in electronegativity between two atoms is large (generally greater than 1.7), the bond is likely ionic, while a smaller difference indicates a covalent bond. This concept is crucial for determining the type of bonding in oxides.
Recommended video:
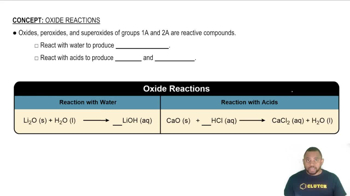
Metallic and Nonmetallic Oxides
Oxides can be classified based on the nature of the elements involved. Metallic oxides, formed from metals and oxygen, tend to be ionic, while nonmetallic oxides, formed from nonmetals and oxygen, are usually molecular. Recognizing the type of elements in the oxide helps in predicting its bonding characteristics.
Recommended video: