(b) Calculate the energy needed for this temperature change.
Consider the data about gold metal in Exercise 5.26(b). (b) Suppose that the same amount of heat is added to two 10.0-g blocks of metal, both initially at the same temperature. One block is gold metal, and one is iron metal. Which block will have the greater rise in temperature after the addition of the heat?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Specific Heat Capacity

Heat Transfer

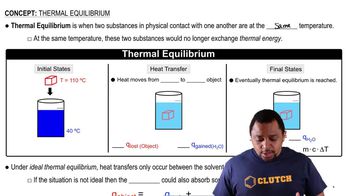
Thermal Equilibrium
The specific heat of octane, C8H18(l), is 2.22 J•g/K. (a) How many J of heat are needed to raise the temperature of 80.0 g of octane from 10.0 to 25.0 °C?
The specific heat of octane, C8H18(l), is 2.22 J•g/K. (b) Which will require more heat, increasing the temperature of 1 mol of C8H18(l), by a certain amount or increasing the temperature of 1 mol of H2O(l) by the same amount?
Consider the data about gold metal in Exercise 5.26(b). (c) What is the molar heat capacity of Au(s)?
When a 6.50-g sample of solid sodium hydroxide dissolves in 100.0 g of water in a coffee-cup calorimeter (Figure 5.18), the temperature rises from 21.6 to 37.8 °C (a) Calculate the quantity of heat (in kJ) released in the reaction.
When a 6.50-g sample of solid sodium hydroxide dissolves in 100.0 g of water in a coffee-cup calorimeter (Figure 5.18), the temperature rises from 21.6 to 37.8 °C (b) Using your result from part (a), calculate H (in kJ/mol KOH) for the solution process. Assume that the specific heat of the solution is the same as that of pure water.
