Specify what ions are present upon dissolving each of the following substances in water: (c) HClO4
Formic acid, HCOOH, is a weak electrolyte. Write the chemical equation for the ionization of HCOOH.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
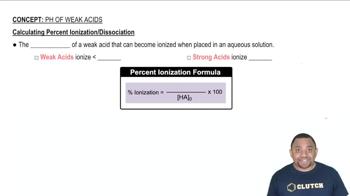
Weak Electrolytes

Ionization of Acids
Chemical Equations

Specify what ions are present upon dissolving each of the following substances in water: (d) NaCH3COO
Formic acid, HCOOH, is a weak electrolyte. What solutes are present in an aqueous solution of this compound?
Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound? What solute particles are present in an aqueous solution of CH3COCH3?
Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound? What solute particles are present in an aqueous solution of HClO?
Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound? What solute particles are present in an aqueous solution of NH4Cl?
