A 1.248-g sample of limestone rock is pulverized and then treated with 30.00 mL of 1.035 M HCl solution. The excess acid then requires 11.56 mL of 1.010 M NaOH for neutralization. Calculate the percentage by mass of calcium carbonate in the rock, assuming that it is the only substance reacting with the HCl solution.
The accompanying photo shows the reaction between a solution of Cd(NO3)2 and one of Na2S. (d) Is this a redox reaction?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Redox Reactions

Oxidation States

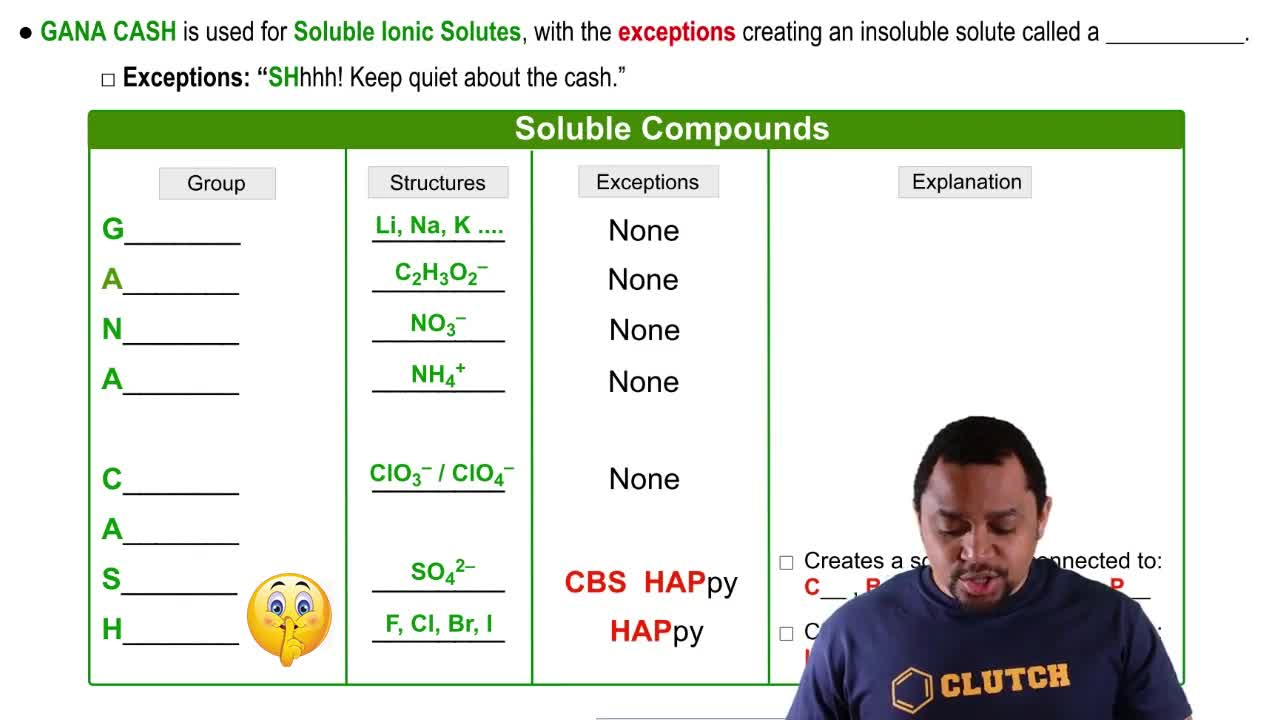
Ionic Compounds and Solubility
Uranium hexafluoride, UF6, is processed to produce fuel for nuclear reactors and nuclear weapons. UF6 can be produced in a two-step reaction. Solid uranium (IV) oxide, UO2, is first made to react with hydrofluoric acid (HF) solution to form solid UF4 with water as a by-product. UF4 further reacts with fluorine gas to form UF6. (a) Write the balanced molecular equations for the conversion of UO2 into UF4 and the conversion of UF4 to UF6. (b) Which step is an acid-base reaction?
The accompanying photo shows the reaction between a solution of Cd(NO3)2 and one of Na2S. (b) What ions remain in solution?
