Consider the following reagents: zinc, copper, mercury (density 13.6 g/mL), silver nitrate solution, nitric acid solution. (a) Given a 500-mL Erlenmeyer flask and a balloon, can you combine two or more of the foregoing reagents to initiate a chemical reaction that will inflate the balloon? Write a balanced chemical equation to represent this process. What is the identity of the substance that inflates the balloon?
Bronze is a solid solution of Cu(s) and Sn(s); solutions of metals like this that are solids are called alloys. There is a range of compositions over which the solution is considered a bronze. Bronzes are stronger and harder than either copper or tin alone. (c) Suggest a reaction that you could do to remove all the tin from this bronze to leave a pure copper sample. Justify your reasoning.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
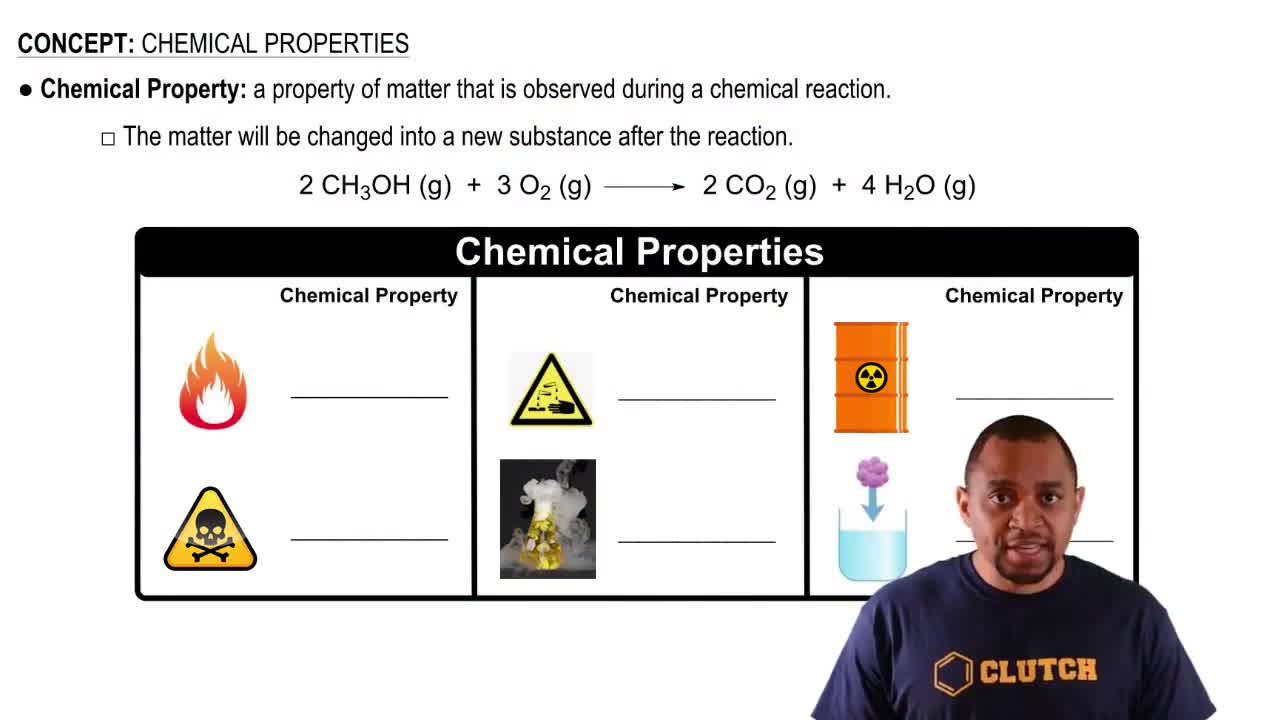
Key Concepts
Alloys
Chemical Reactions
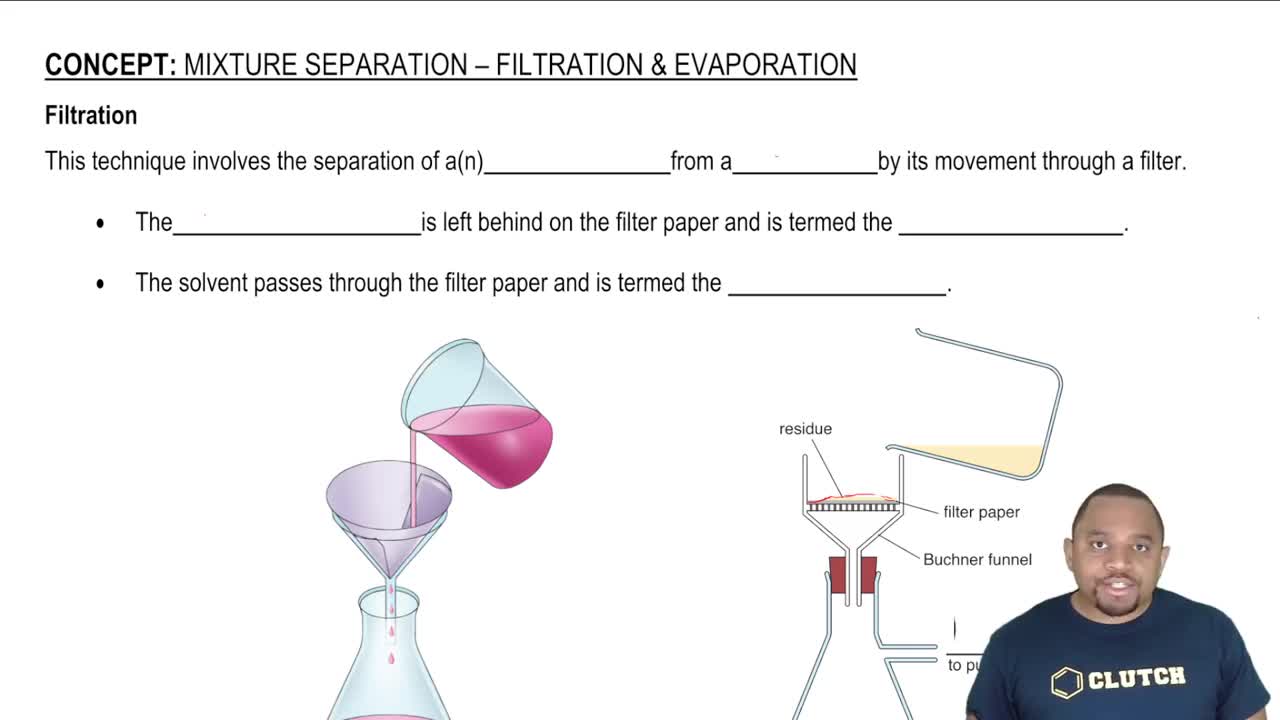
Separation Techniques
Bronze is a solid solution of Cu(s) and Sn(s); solutions of metals like this that are solids are called alloys. There is a range of compositions over which the solution is considered a bronze. Bronzes are stronger and harder than either copper or tin alone. (b) Based on part (a), calculate the concentration of the solute metal in the alloy in units of molarity, assuming a density of 7.9 g/cm3.
Neurotransmitters are molecules that are released by nerve cells to other cells in our bodies, and are needed for muscle motion, thinking, feeling, and memory. Dopamine is a common neurotransmitter in the human brain. (c) Experiments with rats show that if rats are dosed with 3.0 mg/kg of cocaine (that is, 3.0 mg cocaine per kg of animal mass), the concentration of dopamine in their brains increases by 0.75 mM after 60 seconds. Calculate how many molecules of dopamine would be produced in a rat (average brain volume 5.00 mm3) after 60 seconds of a 3.0 mg/kg dose of cocaine.
Hard water contains Ca2+, Mg2+, and Fe2+, which interfere with the action of soap and leave an insoluble coating on the insides of containers and pipes when heated. Water softeners replace these ions with Na+. Keep in mind that charge balance must be maintained. (a) If 1500 L of hard water contains 0.020 M Ca2+ and 0.0040 M Mg2+, how many moles of Na+ are needed to replace these ions?
