The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (a) What is the halflife of the decay? [Section 21.4]
Ch.21 - Nuclear Chemistry
Chapter 21, Problem 6d
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (d) What is the product of the decay process? [Section 21.4]
 Verified step by step guidance
Verified step by step guidance1
Identify the initial isotope undergoing decay: ^{88}_{42}Mo.
Determine the type of decay: positron emission (β^+ decay).
Write the nuclear equation for positron emission: ^{88}_{42}Mo → ^{88}_{41}Nb + β^+.
Identify the product of the decay process: the new element formed is Niobium (Nb) with a mass number of 88 and an atomic number of 41.
Verify the conservation of mass and atomic numbers in the nuclear equation to ensure the correctness of the product.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This can occur through various modes, including alpha decay, beta decay, and positron emission. In the case of positron emission, a proton in the nucleus is transformed into a neutron, releasing a positron and a neutrino, which results in a decrease in the atomic number of the element.
Recommended video:
Guided course

Rate of Radioactive Decay
Decay Products
The decay product is the new element or isotope formed as a result of radioactive decay. For example, when molybdenum-88 (
88
42Mo) undergoes positron emission, it transforms into a different element with a lower atomic number. Understanding the decay products is crucial for applications in nuclear chemistry, medicine, and radiometric dating.
Recommended video:
Guided course

Beta Decay
Half-Life
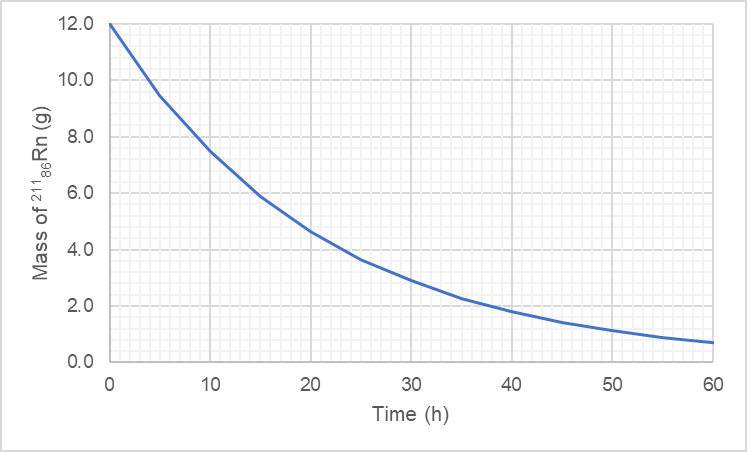
Half-life is the time required for half of the radioactive nuclei in a sample to decay. This concept is essential for predicting the behavior of radioactive substances over time. The graph provided illustrates the decay of radon-218, showing how the mass decreases exponentially, which is characteristic of half-life behavior in radioactive decay processes.
Recommended video:
Guided course

Zero-Order Half-life
Related Practice
Textbook Question
580
views
Textbook Question
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (b) What is the rate constant for the decay? [Section 21.4]
350
views
Textbook Question
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (c) What fraction of the original sample of 8842Mo remains after 12 min? [Section 21.4]
495
views
Open Question
A 65-kg person is accidentally exposed for 240 s to a 15-mCi source of beta radiation coming from a sample of 90Sr. (a) What is the activity of the radiation source in disintegrations per second? In becquerels? (b) Each beta particle has an energy of 8.75 * 10^-14 J, and 7.5% of the radiation is absorbed by the person. Assuming that the absorbed radiation is spread over the person’s entire body, calculate the absorbed dose in rads and in grays. (c) If the RBE of the beta particles is 1.0, what is the effective dose in mrem and in sieverts? (d) Is the radiation dose equal to, greater than, or less than that for a typical mammogram (300 mrem)?
Textbook Question
All the stable isotopes of boron, carbon, nitrogen, oxygen, and fluorine are shown in the accompanying chart (in red), along with their radioactive isotopes with t1>2 7 1 min (in blue). (b) Which radioactive isotopes are most likely to decay by beta emission? [Sections 21.2, 21.4, and 21.5]
844
views
Open Question
A sample of an alpha emitter having an activity of 0.18 Ci is stored in a 25.0-mL sealed container at 22 _x001D_C for 245 days. (b) Assuming that each alpha particle is converted to a helium atom, what is the partial pressure of helium gas in the container after this 245-day period?
