What particle is produced during the following decay processes: (c) iodine-122 decays to xenon-122?
Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: (a) tritium, 31H.

Verified Solution
Key Concepts
Beta Decay

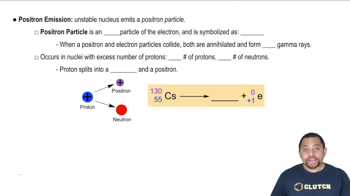
Positron Emission
Tritium

Predict the type of radioactive decay process for the following radionuclides: (a) 85B.
Predict the type of radioactive decay process for the following radionuclides: (b) 6829Cu.
Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: (b) 8938Sr.
Despite the similarities in the chemical reactivity of elements in the lanthanide series, their abundances in Earth's crust vary by two orders of magnitude. This graph shows the relative abundance as a function of atomic number. Which of the following statements best explains the sawtooth variation across the series? (a) The elements with an odd atomic number lie above the belt of stability. (b) The elements with an odd atomic number lie below the belt of stability. (c) The elements with an even atomic number have a magic number of protons. (d) Pairs of protons have a special stability.
Which of the following nuclides would you expect to be radioactive: 5826Fe, 6027Co, 9241Nb, mercury-202, radium-226? Justify your choices.
