Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In this process, an electric current is passed through an electrolyte solution, causing the ions to migrate towards the electrodes, where they undergo reduction or oxidation. For the Cr3+ ions in the solution, electrolysis will result in the deposition of chromium metal at the cathode.
Recommended video:
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis quantify the relationship between the amount of substance transformed at an electrode and the quantity of electric charge passed through the electrolyte. The first law states that the mass of a substance deposited or liberated at an electrode is directly proportional to the total electric charge passed. This relationship is crucial for calculating the mass of chromium plated out in the given electrolysis scenario.
Recommended video:
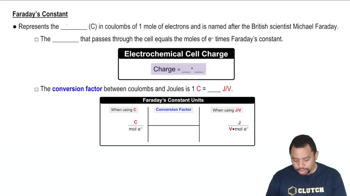
Faraday's Constant in Electrochemistry
Current and Time Relationship
The relationship between current, time, and charge is fundamental in electrolysis calculations. The total charge (Q) can be calculated using the formula Q = I × t, where I is the current in amperes and t is the time in seconds. This charge is then used in conjunction with Faraday's laws to determine the mass of the substance deposited, making it essential for solving the problem of how much chromium is plated out.
Recommended video:
Electrochemical Stoichiometric Chart (Time)
 Verified step by step guidance
Verified step by step guidance