Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when atoms transfer electrons, resulting in the formation of charged ions. These compounds typically consist of a metal and a non-metal, where the metal donates electrons to become a positively charged cation, and the non-metal accepts electrons to become a negatively charged anion. The electrostatic attraction between these oppositely charged ions results in a stable ionic lattice structure.
Recommended video:
Molecular Compounds
Molecular compounds are formed when two or more non-metal atoms share electrons through covalent bonds. Unlike ionic compounds, they do not consist of ions but rather discrete molecules. These compounds often have lower melting and boiling points compared to ionic compounds and can exist in various states (solid, liquid, gas) at room temperature.
Recommended video:
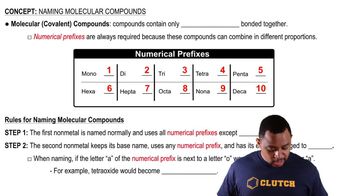
Naming Molecular Compounds
Nitrate Ion
The nitrate ion (NO3-) is a polyatomic ion commonly found in ionic compounds. It consists of one nitrogen atom covalently bonded to three oxygen atoms, with an overall negative charge. In the context of the compound Ca(NO3)2, calcium (Ca) acts as a cation, while the nitrate ions serve as anions, illustrating the formation of an ionic compound through the combination of these charged species.
Recommended video:
 Verified step by step guidance
Verified step by step guidance