There are two different isotopes of bromine atoms. Under normal conditions, elemental bromine consists of Br2 molecules, and the mass of a Br2 molecule is the sum of the masses of the two atoms in the molecule. The mass spectrum of Br2 consists of three peaks: Mass (u) Relative Size 157.836 0.2569 159.834 0.4999 161.832 0.2431 (c) Determine the average molecular mass of a Br2 molecule.
It is common in mass spectrometry to assume that the mass of a cation is the same as that of its parent atom. (b) What percentage of the mass of an 1H atom does the electron represent?
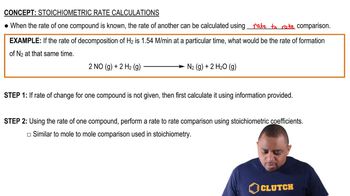
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Mass Spectrometry

Atomic Mass and Electron Mass

Percentage Calculation
There are two different isotopes of bromine atoms. Under normal conditions, elemental bromine consists of Br2 molecules, and the mass of a Br2 molecule is the sum of the masses of the two atoms in the molecule. The mass spectrum of Br2 consists of three peaks: Mass (u) Relative Size 157.836 0.2569 159.834 0.4999 161.832 0.2431 (d) Determine the average atomic mass of a bromine atom
There are two different isotopes of bromine atoms. Under normal conditions, elemental bromine consists of Br2 molecules, and the mass of a Br2 molecule is the sum of the masses of the two atoms in the molecule. The mass spectrum of Br2 consists of three peaks: Mass (u) Relative Size 157.836 0.2569 159.834 0.4999 161.832 0.2431 (e) Calculate the abundances of the two isotopes. Calculate the abundance of the heavier isotope.
From the following list of elements—Mg, Li, Tl, Pb, Se, Cl, Xe, Si, C—pick the one that best fits each description. Use each element only once: (a) an alkali metal, (b) an alkaline earth metal, (c) a noble gas, (d) a halogen, (e) a metalloidin group 14, (f) a nonmetal listed in group 14, (g) a metal that forms a 3+ ion, (h) a nonmetal that forms a 2- ion, (i) an element that is used as radiation shielding.
The first atoms of seaborgium (Sg) were identified in 1974. The longest-lived isotope of Sg has a mass number of 266. (a) How many protons, electrons, and neutrons are in an 266Sg atom?
The first atoms of seaborgium (Sg) were identified in 1974. The longest-lived isotope of Sg has a mass number of 266. (b) Atoms of Sg are very unstable, and it is therefore difficult to study this element's properties. Based on the position of Sg in the periodic table, what element should it most closely resemble in its chemical properties?
