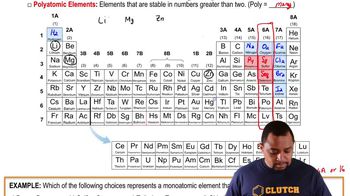
Here are the essential concepts you must grasp in order to answer the question correctly.
Isotopes
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. This means they occupy the same position on the periodic table and have the same atomic number, but their mass numbers differ. For example, carbon-12 and carbon-14 are isotopes of carbon, differing in their neutron count.
Recommended video:
Atomic Number and Mass Number
The atomic number of an element is the number of protons in its nucleus, which defines the element itself. The mass number is the total number of protons and neutrons in the nucleus. In the notation <sup>A</sup><sub>Z</sub>X, A represents the mass number and Z represents the atomic number, helping to identify isotopes based on their mass differences.
Recommended video:
Element Identification
Elements are identified by their atomic number, which corresponds to the number of protons. Isotopes of the same element will have the same atomic number but different mass numbers. In the given question, identifying which isotopes belong to the same element involves comparing their atomic numbers to determine if they are the same.
Recommended video:
Chalcogen Identification Example
 Verified step by step guidance
Verified step by step guidance