Textbook Question
Identify the Lewis acid and Lewis base in each of the following reactions: (b) FeBr31s2 + Br-1aq2 Δ FeBr4-1aq2
1152
views
 Verified step by step guidance
Verified step by step guidance


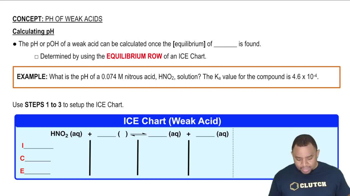
Identify the Lewis acid and Lewis base in each of the following reactions: (b) FeBr31s2 + Br-1aq2 Δ FeBr4-1aq2
Which member of each pair produces the more acidic aqueous solution: (b) CuCl or Cu1NO322
Indicate whether each of the following statements is correct or incorrect. (d) K+ ion is acidic in water because it causes hydrating water molecules to become more acidic.
A solution is made by adding 0.300 g Ca1OH221s2, 50.0 mL of 1.40 M HNO3, and enough water to make a final volume of 75.0 mL. Assuming that all of the solid dissolves, what is the pH of the final solution?