Textbook Question
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) Cl2(g) → 2 Cl(g)
453
views



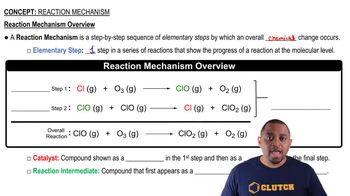
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) Cl2(g) → 2 Cl(g)
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (b) OCl-(aq + H2O(l) → HOCl(aq) + OH-(aq)
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (c) NO(g) + Cl2(g) → NOCl2(g)
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (b)
(a) Based on the following reaction profile, how many intermediates are formed in the reaction A→D?
(c) Which step is the fastest?