(b) How can you calculate the rate constant for a first-order reaction from the graph you made in part (a)?
(b) At 320°C the rate constant is 2.2 × 10-5 s-1. What is the half-life at this temperature?
Recommended similar problem, with video answer:

Verified Solution
Key Concepts
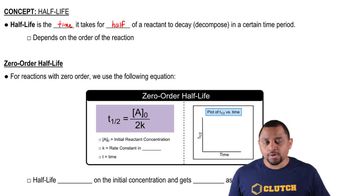
Rate Constant

Half-Life
First-Order Kinetics

The decomposition of sodium bicarbonate (baking soda), NaHCO3(s), into Na2CO3(s), H2O(l), and CO2(g) at constant pressure requires the addition of 85 kJ of heat per two moles of NaHCO3. (b) Draw an enthalpy diagram for the reaction.
(a) The gas-phase decomposition of SO2Cl2, SO2Cl2(g) → SO2(g) + Cl2(g), is first order in SO2Cl2. At 600 K the half-life for this process is 2.3 × 105 s. What is the rate constant at this temperature?
As described in Exercise 14.41, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 × 10-2 s-1. (a) If we begin with an initial SO2Cl2 pressure of 450 torr, what is the partial pressure of this substance after 60 s?
As described in Exercise 14.41, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 × 10-2 s-1. (b) At what time will the partial pressure of SO2Cl2 decline to one-tenth its initial value?
