Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (b) 0.240 mol of naphthalene (C10H8) in 2.45 mol of chloroform,
Ch.13 - Properties of Solutions
Chapter 13, Problem 76
What is the freezing point of an aqueous solution that boils at 105.0 °C?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Determine the boiling point elevation (ΔT_b) using the formula ΔT_b = T_b(solution) - T_b(pure solvent), where T_b(solution) is 105.0 °C and T_b(pure solvent) is 100.0 °C for water.
insert step 2> Use the boiling point elevation formula ΔT_b = i * K_b * m, where i is the van't Hoff factor, K_b is the ebullioscopic constant for water, and m is the molality of the solution. Solve for m.
insert step 3> Use the calculated molality (m) from step 2 in the freezing point depression formula ΔT_f = i * K_f * m, where K_f is the cryoscopic constant for water.
insert step 4> Calculate the freezing point depression (ΔT_f) using the formula from step 3.
insert step 5> Determine the freezing point of the solution by subtracting the freezing point depression (ΔT_f) from the freezing point of pure water (0.0 °C).

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Colligative Properties
Colligative properties are physical properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. These properties include boiling point elevation and freezing point depression, which are crucial for understanding how solutes affect the phase changes of solvents.
Recommended video:
Guided course
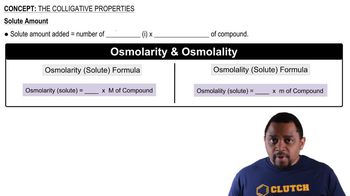
Colligative Properties
Boiling Point Elevation
Boiling point elevation occurs when a non-volatile solute is added to a solvent, resulting in an increase in the boiling point of the solution compared to the pure solvent. This phenomenon can be quantified using the formula ΔT_b = i * K_b * m, where ΔT_b is the boiling point elevation, i is the van 't Hoff factor, K_b is the ebullioscopic constant, and m is the molality of the solution.
Recommended video:
Guided course

Boiling Point Elevation
Freezing Point Depression
Freezing point depression is the decrease in the freezing point of a solvent when a solute is dissolved in it. Similar to boiling point elevation, it can be calculated using the formula ΔT_f = i * K_f * m, where ΔT_f is the freezing point depression, i is the van 't Hoff factor, K_f is the cryoscopic constant, and m is the molality of the solution. This concept is essential for determining the freezing point of the solution in the given question.
Recommended video:
Guided course

Freezing Point Depression
Related Practice
Textbook Question
1130
views
Textbook Question
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3;
1576
views
Open Question
How many grams of ethylene glycol (C2H6O2) must be added to 1.00 kg of water to produce a solution that freezes at -5.00 °C?
Open Question
What is the osmotic pressure formed by dissolving 44.2 mg of aspirin (C9H8O4) in 0.358 L of water at 25 °C?
Textbook Question
Seawater contains 34 g of salts for every liter of solution. Assuming that the solute consists entirely of NaCl (in fact, over 90% of the salt is indeed NaCl), calculate the osmotic pressure of seawater at 20 °C
832
views
Textbook Question
Adrenaline is the hormone that triggers the release of extra glucose molecules in times of stress or emergency. A solution of 0.64 g of adrenaline in 36.0 g of CCl4 elevates the boiling point by 0.49 °C. Calculate the approximate molar mass of adrenaline from this data.
844
views
