Indicate the type of solute–solvent interaction (Section 11.2) that should be most important in each of the following solutions: (b) methanol (CH3OH) in water
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (a) KCl in water
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Ion-Dipole Interactions

Solvation
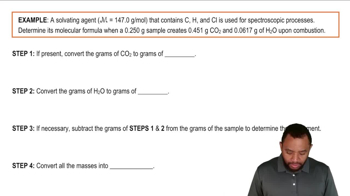
Ranking Solute-Solvent Interactions

Indicate the type of solute–solvent interaction (Section 11.2) that should be most important in each of the following solutions: (c) KBr in water
Indicate the type of solute–solvent interaction (Section 11.2) that should be most important in each of the following solutions: (d) HCl in acetonitrile (CH3CN)
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (b) CH2Cl2 in benzene (C6H6)
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
An ionic compound has a very negative ∆Hsoln in water (b) Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?
