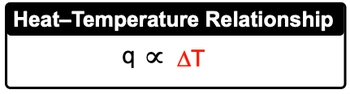
Now heat capacity, which uses capital C, is the amount of heat required to change the temperature of a weighted substance. The more heat that's applied to a substance, the greater its temperature change. Now it can be looked at also in terms of specific heat capacity and molar heat capacity.
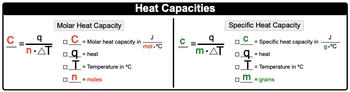
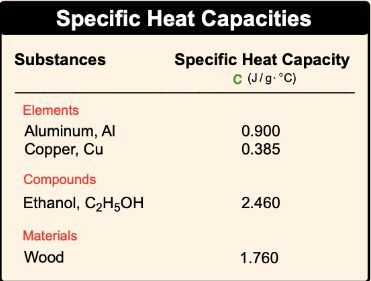
With specific heat capacity, we use lower case c, and it is the amount of heat required to change the temperature of 1g of a substance by 1�. That degree can be either in Kelvin or degrees Celsius. Here, molar heat capacity is just like heat capacity in terms of it being a capital C, but here with molar heat capacity, it's the amount of heat required to change the temperature of 1 mole of a substance by 1� either in Kelvin or degrees Celsius.
OK, so think of molar heat capacity as being a little bit more focused in terms of the way we look at heat capacity in terms of 1 mole of a substance. Now we're going to say here that when it comes to molar heat capacity, which is capital C, it equals QNΔT. So capital C equals our molar heat capacity in Joules over moles times degrees Celsius or in Kelvin. Q represents heat, T equals temperature in degrees Celsius.
But what we need to realize here is that whatever the units that the molar heat capacity uses for temperature, temperature should match it. OK, so if this happened to be in Kelvin, then temperature should be in Kelvin, and then N is equal to our moles. With our specific heat capacity, it uses lower case c, it equals QmΔT. Here, lowercase c is our specific heat capacity in joules over grams times degrees Celsius or Kelvin.
Q again is heat, temperature again can be in Celsius or in Kelvin. To determine which one to use, you look at the units for your specific heat capacity and make sure they match, and then lower case m here is just grams of our substance. So just remember the difference between molar heat capacity and specific heat capacity.