Stoichiometric Rate Calculations definitions Flashcards
 Back
BackStoichiometric Rate Calculations definitions
1/10
Terms in this set (10)
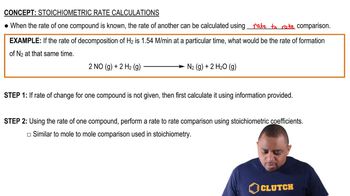
- Stoichiometric Rate CalculationsDetermining the rate of one compound's formation or decomposition using another's rate and stoichiometric coefficients.
- Rate-to-Rate ComparisonA method to compare the rates of two compounds using their stoichiometric coefficients in a balanced equation.
- Stoichiometric CoefficientsNumbers in a balanced chemical equation indicating the ratio of moles of each substance involved.
- Decomposition RateThe speed at which a compound breaks down into simpler substances, measured in molarity per minute.
- Formation RateThe speed at which a compound is produced in a reaction, typically measured in molarity per minute.
- Balanced Chemical EquationAn equation with equal numbers of each type of atom on both sides, reflecting the conservation of mass.
- MolarityA concentration unit representing moles of solute per liter of solution.
- Nitrogen GasA diatomic molecule, N2, often involved in chemical reactions as a product or reactant.
- H2Molecular hydrogen, a diatomic molecule often involved in reactions as a reactant or product.
- Mole-to-Mole ComparisonA stoichiometric method comparing moles of reactants and products using coefficients from a balanced equation.