Types of Aqueous Solutions definitions Flashcards
 Back
BackTypes of Aqueous Solutions definitions
1/12
Terms in this set (12)
- Solution EquilibriumA state where the rates of solute dissolution and recrystallization are equal.
- DissolutionThe process where a solid solute breaks into ions in a solvent.
- RecrystallizationThe process where dissolved solute reforms into a solid.
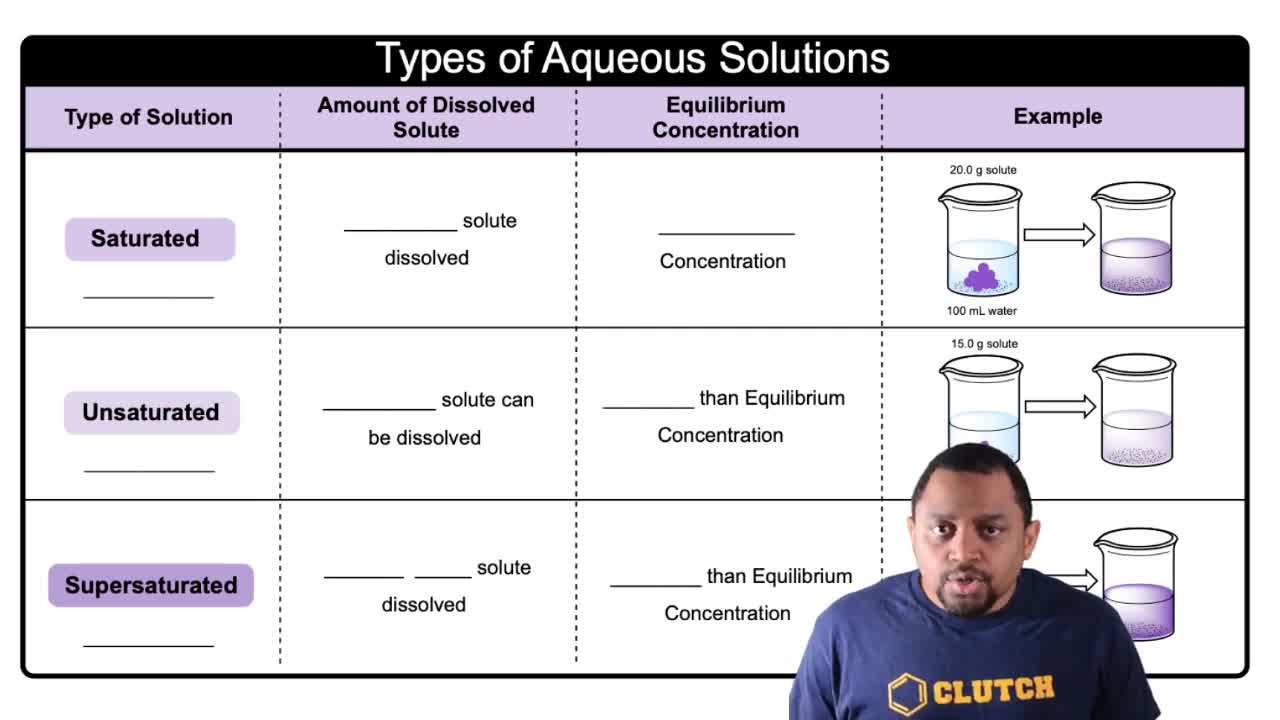
- Saturated SolutionA stable solution with the maximum amount of solute dissolved at equilibrium.
- Unsaturated SolutionA stable solution that can dissolve more solute, below equilibrium concentration.
- Supersaturated SolutionAn unstable solution with more solute than equilibrium allows, often requiring heat.
- Equilibrium ConcentrationThe maximum solute concentration in a solution at a given temperature.
- Homogeneous MixtureA mixture with uniform composition throughout, such as a solution.
- SoluteThe substance dissolved in a solvent to form a solution.
- SolventThe substance in which a solute is dissolved to form a solution.
- TemperatureA factor that can increase solubility, affecting solution saturation.
- SolubilityThe ability of a solute to dissolve in a solvent at a specific temperature.