Gibbs Free Energy definitions Flashcards
 Back
BackGibbs Free Energy definitions
1/10
Terms in this set (10)
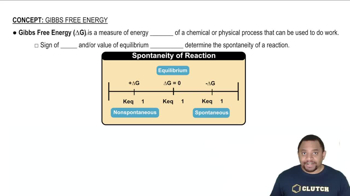
- Gibbs Free EnergyA measure of the energy change in a process that indicates its ability to perform work.
- SpontaneityA process characteristic where it occurs naturally without external energy input, indicated by a negative ΔG.
- EquilibriumA state where ΔG is zero, indicating no net change in the concentrations of reactants and products.
- Equilibrium ConstantA ratio of product concentrations to reactant concentrations at equilibrium, denoted as K.
- Non-SpontaneousA process that requires energy input to occur, indicated by a positive ΔG.
- EnthalpyA thermodynamic quantity represented by ΔH, related to the heat content of a system.
- EntropyA measure of disorder or randomness in a system, represented by ΔS.
- TemperatureA factor that influences the spontaneity of a reaction, affecting the signs of ΔH and ΔS.
- ProductsSubstances formed as a result of a chemical reaction, influencing the value of K.
- ReactantsSubstances consumed during a chemical reaction, influencing the value of K.