Constant-Pressure Calorimetry definitions Flashcards
 Back
BackConstant-Pressure Calorimetry definitions
1/15
Terms in this set (15)
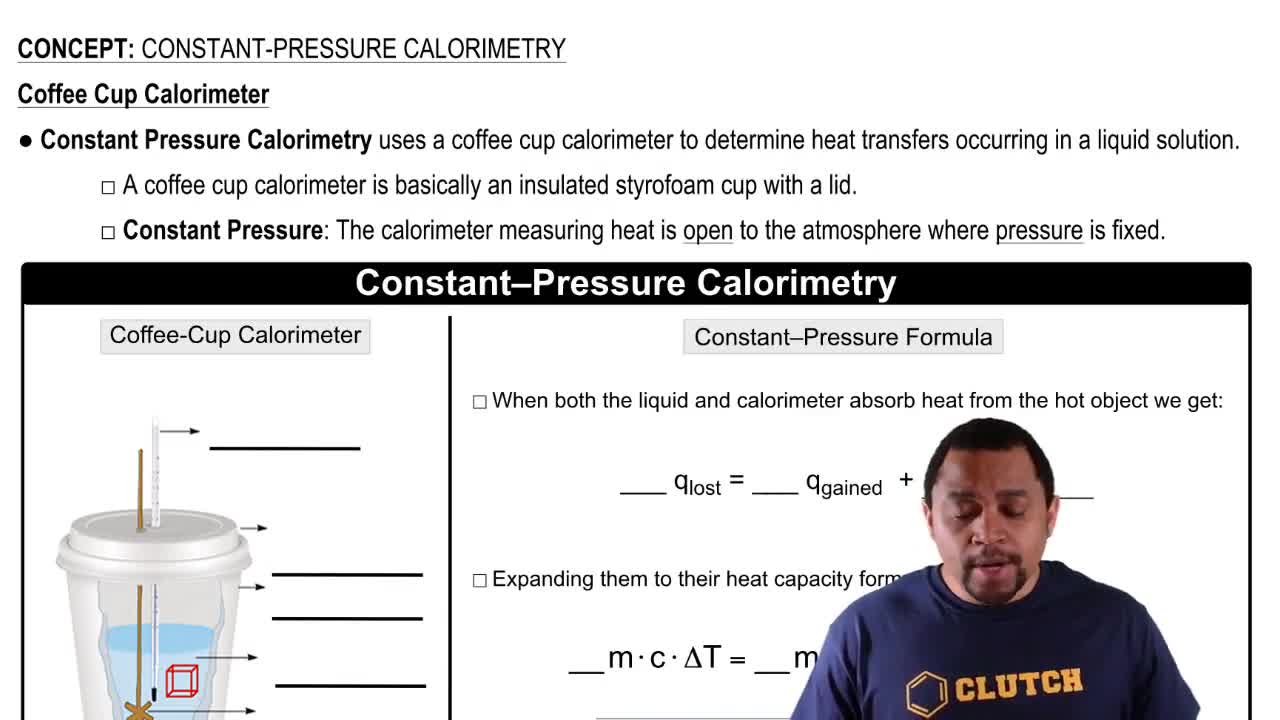
- CalorimeterAn insulated container used to measure heat transfer during chemical or physical processes.
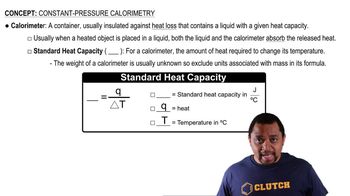
- Heat CapacityThe amount of heat required to change the temperature of an object by one degree Celsius.
- Coffee Cup CalorimeterAn insulated styrofoam cup used in constant pressure calorimetry to measure heat transfer.
- Atmospheric PressureThe pressure exerted by the weight of the atmosphere, under which constant pressure calorimetry occurs.
- Specific Heat CapacityThe heat required to raise the temperature of one gram of a substance by one degree Celsius.
- Temperature ChangeThe difference in temperature before and after a heat transfer process.
- JoulesA unit of energy used to quantify heat transfer in calorimetry.
- KelvinA temperature scale used in scientific measurements, where 0 K is absolute zero.
- StyrofoamA material used in coffee cup calorimeters for insulation to minimize heat loss.
- ThermometerA device used to measure temperature changes in a calorimeter.
- StirrerA tool used in a calorimeter to ensure uniform temperature distribution in the liquid.
- Heat TransferThe movement of thermal energy from a hotter object to a cooler one.
- MassThe quantity of matter in an object, used in calculating heat transfer.
- Degrees CelsiusA temperature scale where water freezes at 0° and boils at 100° under standard conditions.
- KilojoulesA unit of energy equal to 1,000 joules, often used in calorimetry.