Clausius-Clapeyron Equation definitions Flashcards
 Back
BackClausius-Clapeyron Equation definitions
1/15
Terms in this set (15)
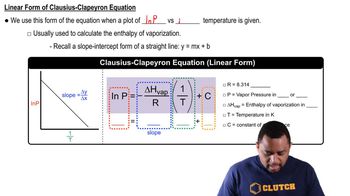
- Clausius-Clapeyron EquationDescribes the relationship between vapor pressure and temperature, showing that vapor pressure increases with temperature.
- Vapor PressureThe pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature.
- Enthalpy of VaporizationThe heat required to convert a liquid into a gas at constant temperature and pressure.
- Gas ConstantA physical constant denoted as R, with a value of 8.314 J/mol·K, used in various equations of state.
- Natural LogarithmThe logarithm to the base e, where e is an irrational constant approximately equal to 2.71828.
- Inverse TemperatureThe reciprocal of temperature, often used in thermodynamic equations, represented as 1/T.
- SlopeIn the context of the Clausius-Clapeyron equation, it represents -ΔHvap/R in a plot of ln P versus 1/T.
- Normal Boiling PointThe temperature at which a liquid's vapor pressure equals 760 torr, allowing it to boil under normal atmospheric pressure.
- TorrA unit of pressure equivalent to 1/760 of an atmosphere, commonly used in measuring vapor pressure.
- KelvinThe SI base unit of temperature, where 0 K is absolute zero, used in scientific temperature measurements.
- Linear FormA representation of the Clausius-Clapeyron equation as a straight line, useful for plotting ln P against 1/T.
- Two-Point FormA form of the Clausius-Clapeyron equation used when comparing two sets of temperatures and pressures.
- EquilibriumA state where the rate of vaporization equals the rate of condensation, resulting in a constant vapor pressure.
- CondensationThe process by which a gas transforms into a liquid, often occurring when vapor pressure decreases.
- VaporizationThe process of converting a liquid into a gas, typically requiring heat input to overcome intermolecular forces.