10. Periodic Properties of the Elements
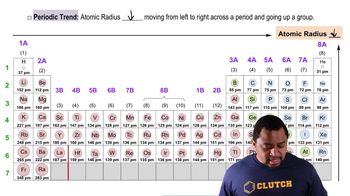
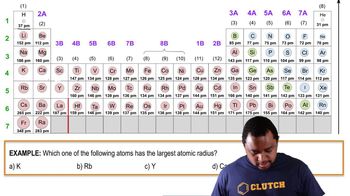
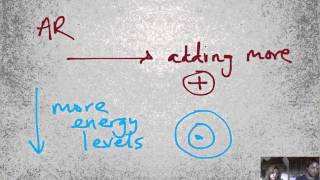
Periodic Trend: Atomic Radius
10. Periodic Properties of the Elements
Periodic Trend: Atomic Radius
Additional 5 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
Showing 30 of 30 practice