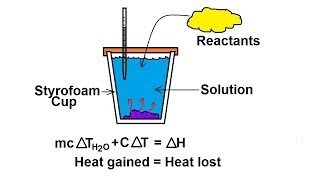
8. Thermochemistry
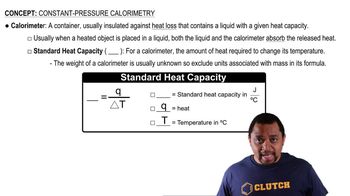
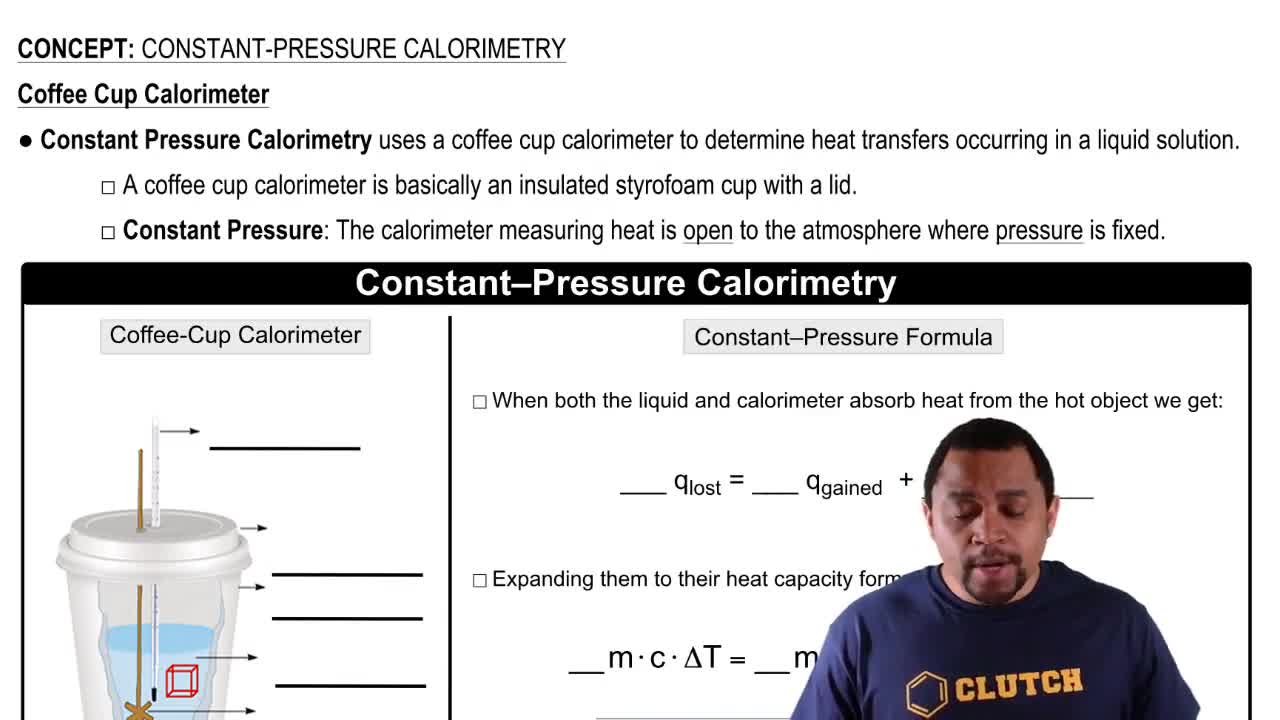
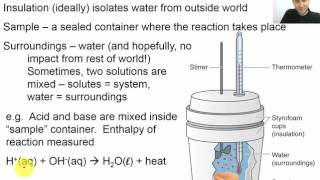
Constant-Pressure Calorimetry
8. Thermochemistry
Constant-Pressure Calorimetry
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
A 115.6 g piece of copper metal at 182.5 ºC is placed into 120.0 mL of methylene chloride at 31.0 ºC within a coffee-cup calorimeter. If the final temperature of the solution is 50.3 ºC, what is the specific heat of methylene chloride? Assume the calorimeter absorbs a negligible amount of heat. The specific heat of copper is 4.184 J/g ∙ ºC and the density of methylene chloride is 1.33 g/cm3.
1026views1rank2comments - Multiple Choice
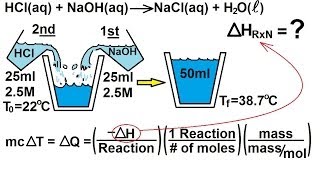
You place 75.0 mL of 0.100 M NaOH in a calorimeter at 25.00 ºC and carefully add 55.0 mL of 0.200 M HNO3, also at 25.00 ºC. After stirring, the final temperature is 53.35 ºC. Calculate the enthalpy ∆Hrxn (in J/mol) for the formation of water. (Specific heat capacity, Cs, and density of the solution:4.184 J/g∙K and 1.00 g/mL).
1327views6rank12comments - Multiple ChoiceWhen gasoline is burned at constant pressure, a certain amount of heat is emitted and work is done. Which of the following must be true?334views
- Open QuestionWhen a 4.25g sample of solid ammonium398views