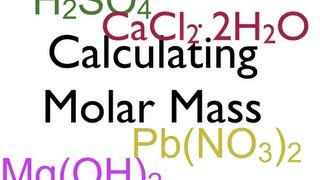2. Atoms & Elements
Calculating Molar Mass
2. Atoms & Elements
Calculating Molar Mass
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Calculate the molecular weight of C3H5N3O3.
3025views30rank2comments - Multiple Choice
The reaction between nickel metal and hydrochloric acid is not a simple dissolution. The product formed is NiCl2 • 6 H2O (s), nickel (II) chloride hexahydrate, which has exactly 6 waters of hydration in the crystal lattice for every nickel ion. What is the molar mass of nickel (II) chloride hexahydrate, NiCl2 • 6 H2O (s)?
4955views20rank1comments - Multiple Choice
What is the molar mass of diazepam also known as Valium if 0.05570 mol weighs 15.86 g?
3579views24rank1comments - Multiple ChoiceUse the data in the table to calculate the atomic mass of silicon.
Exact Mass and Abundance of 3 Isotopes of Silicon Isotope Isotopic Mass Abundance 28Si 27.97693 amu 92.23% 29Si 28.97649 amu 4.67% 30Si 29.97376 amu 3.10% 1095views - Open Question
What is the molar mass of CHCl3? 48.47 g/mol, 83.92 g/mol, 119.37 g/mol, or 121.39 g/mol?
963views - Open Question
Which is the molar mass of H2O? 10.02 g/mol 16.00 g/mol 17.01 g/mol 18.02 g/mol
859views - Open Question
Here is the query: What is the molar mass of fluorine, F2? Is it 9.00 g/mol, 18.00 g/mol, 19.00 g/mol, or 38.00 g/mol?
1137views - Open QuestionCalculate the molar mass of each compound. keep at least one decimal place in atomic masses from the periodic table.929views