17. Acid and Base Equilibrium
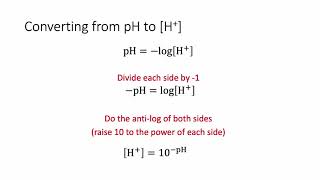
The pH Scale
17. Acid and Base Equilibrium
The pH Scale
Showing 6 of 6 videos
Additional 1 creators.
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following solutions will have the lowest concentration of hydronium ions?
1605views2rank6comments - Multiple Choice
Which of the following statements about aqueous solutions is/are true?
4269views3rank - Multiple Choice
What is the Kw of pure water at 20.0°C, if the pH is 7.083?
3920views7rank5comments - Multiple Choice
Calculate the pH of 50.00 mL of 4.3 x 10-7 M H2SO4.
2329views4rank5comments - Open Question
The pH of an aqueous solution is 4.32. What is the [OH–]?
716views - Open Question
What are the concentrations of H3O+ and OH− in tomatoes that have a pH of 4.10?
632views - Open Question
A solution of ammonia has a pH of 11.8. What is the concentration of OH– ions in the solution?
619views - Open Question
What is the ratio of H+ ions to OH– ions at a pH = 7?
633views