16. Chemical Equilibrium
ICE Charts
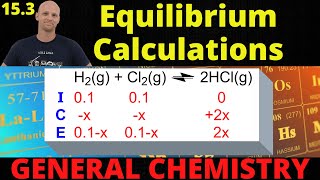
16. Chemical Equilibrium
ICE Charts
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
For the reaction:N2 (g) + 2 O2 (g) ⇌ 2 NO2 (g), Kc = 8.3 x 10 -10 at 25°C. What is the concentration of N2 gas at equilibrium when the concentration of NO2 is twice the concentration of O2 gas?
2915views2rank8comments - Multiple Choice
At a given temperature the gas phase reaction:N2 (g) + O2 (g) ⇌ 2 NO (g) has an equilibrium constant of 4.00 x 10 -15. What will be the concentration of NO at equilibrium if 2.00 moles of nitrogen and 6.00 moles oxygen are allowed to come to equilibrium in a 2.0 L flask.
1927views1rank17comments - Multiple ChoiceCalculate the reaction quotient for the reaction below where the concentrations are [SO3] = 0.500 M, [SO2] = 0.0500 M, and [O2] = 0.100 M.
2 SO3 (g) ⇆ 2 SO2 (g) + O2 (g)
445views - Multiple ChoiceIf Kc for the following reaction is 5.4 × 1013,
2 NO (g) + O2 (g) ⇆ 2 NO2 (g)
what is Kc for the reaction shown below?
4 NO2 (g) ⇆ 4 NO (g) + 2 O2 (g)
453views