If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of b. NO–
Determine the electron configurations for CN+, CN, and CN-. b. Which species, if any, is paramagnetic?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Electron Configuration

Ionic Charge and Electron Count

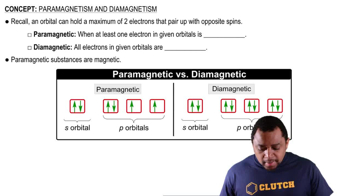
Paramagnetism
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of d. NeF+
Determine the electron configurations for CN+, CN, and CN-. (a) Which species has the strongest C¬N bond?
(c) With what neutral homonuclear diatomic molecules are the NO+ and NO- ions isoelectronic (same number of electrons)? With what neutral homonuclear diatomic molecule is the NO- ion isoelectronic (same number of electrons)?
Assume that the MOs of diatomics from the third row of the periodic table, such as P2, are analogous to those from the second row.
a. Which valence atomic orbitals of P are used to construct the MOs of P2?
Assume that the MOs of diatomics from the third row of the periodic table, such as P2, are analogous to those from the second row.
c. For the P2 molecule, how many electrons occupy the MO in the figure?
