Calculate the pH of solutions prepared by:
(d) Mixing equal volumes of 0.20 M HCl and 0.50 M HNO3.
(Assume that volumes are additive.)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
pH Scale

Strong Acids

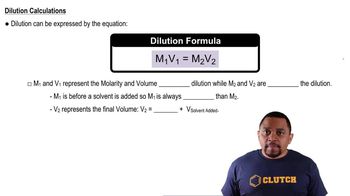
Dilution and Volume Addition
What is the pH and the principal source of H3O+ ions in 1.0 * 10-10 M HCl? (Hint: The pH of an acid solution can’t exceed 7.) What is the pH of 1.0 * 10-7 M HCl?
The acidity of lemon juice is derived primarily from citric acid (H3Cit), a triprotic acid. What are the concentrations of H3Cit, H2Cit-, HCit2-, and Cit3- in a sample of lemon juice that has a pH of 2.37 and a total concentration of the four citrate-containing species of 0.350 M?
A 100.0 mL sample of a solution that is 0.100 M in HCl and 0.100 M in HCN is titrated with 0.100 M NaOH. Calculate the pH after the addition of the following volumes of NaOH:
(a) 0.0 mL
A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equivalence point was reached after 126.4 mL of base.
(e) Sketch the pH titration curve, and label the buffer regions and equivalence points.
