The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (a) What is the halflife of the decay? [Section 21.4]
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (d) What is the product of the decay process? [Section 21.4]
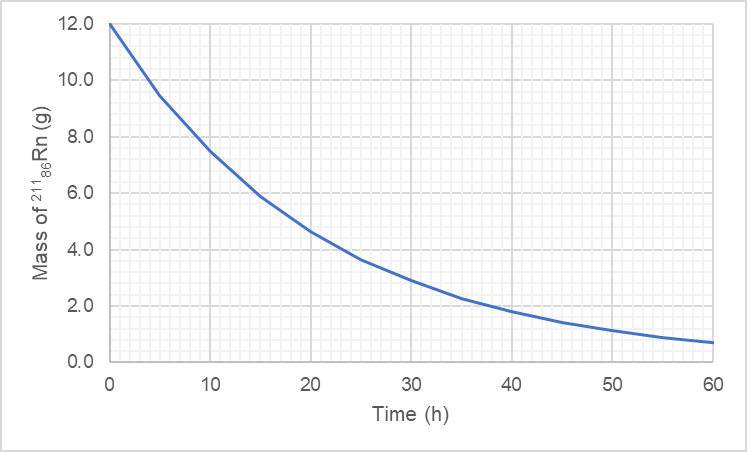

Verified Solution
Key Concepts
Radioactive Decay

Decay Products

Half-Life

The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (b) What is the rate constant for the decay? [Section 21.4]
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (c) What fraction of the original sample of 8842Mo remains after 12 min? [Section 21.4]
All the stable isotopes of boron, carbon, nitrogen, oxygen, and fluorine are shown in the accompanying chart (in red), along with their radioactive isotopes with t1>2 7 1 min (in blue). (b) Which radioactive isotopes are most likely to decay by beta emission? [Sections 21.2, 21.4, and 21.5]
Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.
