Here are the essential concepts you must grasp in order to answer the question correctly.
Ligand Field Theory
Ligand Field Theory explains how ligands affect the energy levels of d-orbitals in transition metal complexes. Strong-field ligands cause a larger splitting of the d-orbitals, leading to lower energy configurations and often resulting in low-spin complexes. Conversely, weak-field ligands cause smaller splitting, leading to high-spin configurations. Understanding this theory is crucial for predicting the magnetic and color properties of coordination complexes.
Recommended video:
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.
Color and Electronic Transitions
The color of a coordination complex is determined by the wavelengths of light absorbed during electronic transitions between d-orbitals. A colorless complex indicates that it does not absorb visible light, which can suggest that the d-orbital splitting is either very small or that the electronic transitions do not fall within the visible spectrum. This concept is essential for understanding the relationship between ligand strength and the resulting color of the complex.
Recommended video:
Electron Configurations Of Transition Metals Example
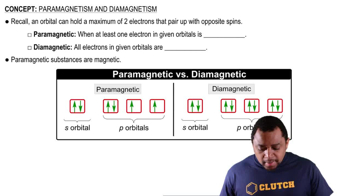
Diamagnetism
Diamagnetism is a property of materials that are not attracted to a magnetic field and occurs when all electrons are paired. In coordination complexes, the presence of paired electrons in the d-orbitals, often due to strong-field ligands, results in diamagnetism. Recognizing the relationship between ligand strength, electron pairing, and magnetic properties helps in predicting the behavior of complexes like [Cr(CO)6].
Recommended video:
Paramagnetism and Diamagnetism