Here are the essential concepts you must grasp in order to answer the question correctly.
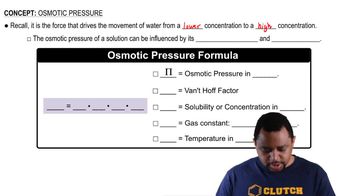
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly proportional to the molarity of the solute particles in the solution, as described by the formula π = iCRT, where π is osmotic pressure, i is the van 't Hoff factor, C is molarity, R is the ideal gas constant, and T is temperature in Kelvin.
Recommended video:
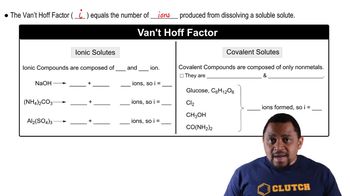
Van 't Hoff Factor (i)
The van 't Hoff factor (i) indicates the number of particles into which a solute dissociates in solution. For glucose, which does not dissociate, i equals 1. This factor is crucial for calculating osmotic pressure, as it affects the total concentration of solute particles contributing to the osmotic effect.
Recommended video:
Ideal Gas Constant (R)
The ideal gas constant (R) is a fundamental constant used in various equations in chemistry, including those involving gases and osmotic pressure. Its value is typically 0.0821 L·atm/(K·mol). In the context of osmotic pressure, it helps relate the pressure, temperature, and molarity of the solution, allowing for the calculation of the required molarity to match a specific osmotic pressure.
Recommended video:
 Verified step by step guidance
Verified step by step guidance