Here are the essential concepts you must grasp in order to answer the question correctly.
Zinc Blende Structure
The zinc blende structure, also known as sphalerite, is a type of crystal lattice where zinc (Zn) cations are tetrahedrally coordinated by sulfur (S) anions. This structure is characterized by a face-centered cubic arrangement of anions with cations occupying half of the tetrahedral sites. Understanding this arrangement is crucial for predicting how additional cations would affect the overall structure.
Recommended video:
Tetrahedral Sites
Tetrahedral sites in a crystal lattice are positions where cations can be located, surrounded by four anions at the corners of a tetrahedron. In the zinc blende structure, each unit cell contains tetrahedral sites that can accommodate additional cations. Filling these sites alters the stoichiometry and can lead to different structural properties, which is essential for understanding the implications of the question.
Recommended video:
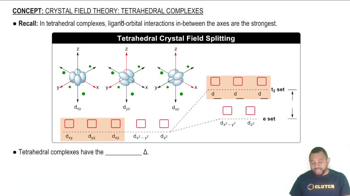
The crystal field splitting pattern for tetrahedral complexes has the d orbitals in between the axes as having the higher energy.
Crystal Structure Modification
When additional cations occupy the tetrahedral sites in a crystal structure, it can lead to a new phase or modification of the existing structure. This can result in changes to the material's properties, such as conductivity, stability, and reactivity. In the case of zinc blende, filling all tetrahedral sites could lead to a structure similar to that of a different compound, such as a spinel structure, which has distinct characteristics.
Recommended video:
 Verified step by step guidance
Verified step by step guidance