Which of these crystal-field splitting diagrams represents:
a. a weak-field octahedral complex of Fe³⁺ ,
b. a strong-field octahedral complex of Fe³⁺
c. a tetrahedral complex of Fe³⁺
d. a tetrahedral complex of Ni²⁺ (The diagrams do not indicate the relative magnitudes of ∆. ) [Find more in Section 23.6.]
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
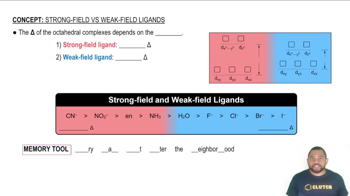
Crystal Field Theory

Octahedral vs. Tetrahedral Complexes

Weak-field vs. Strong-field Ligands
Four-coordinate metals can have either a tetrahedral or a square-planar geometry; both possibilities are shown here for [PtCl2(NH3)2].
a. What is the name of this molecule?
b. Would the tetrahedral molecule have a geometric isomer?
c. Would the tetrahedral molecule be diamagnetic or paramagnetic?
d. Would the square-planar molecule have a geometric isomer?
In the linear crystal-field shown here, the negative charges are on the z-axis. Using Figure 23.28 as a guide, predict which of the following choices most accurately describes the splitting of the d orbitals in a linear crystal-field? [Find more in Section 23.6.]
