(d) When the coffee is brewed according to directions, a pound of coffee beans yields 50 cups of coffee (4 cups = 1 qt). How many kg of coffee are required to produce 200 cups of coffee?
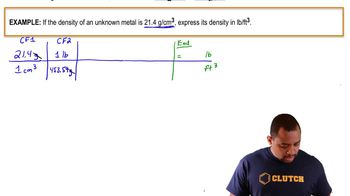
The density of tungsten metal is 19.35 g/cm3.
a. What is the density of tungsten in pounds per cubic foot?
b. A rectangular block of tungsten has a length of 4.00 cm, a width of 2.00 cm, and a height of 1.50 cm. Using the correct number of significant figures, what is the mass of the block in kg?

Verified Solution
Key Concepts
Density Conversion
Volume Calculation

Significant Figures

(a) If an electric car is capable of going 225 km on a single charge, how many charges will it need to travel from Seattle, Washington, to San Diego, California, a distance of 1257 mi, assuming that the trip begins with a full charge?
(d) In March 1989, the Exxon Valdez ran aground and spilled 240,000 barrels of crude petroleum off the coast of Alaska. One barrel of petroleum is equal to 42 gal. How many liters of petroleum were spilled?
Gold can be hammered into extremely thin sheets called gold leaf. An architect wants to cover a 100 ft x 82 ft ceiling with gold leaf that is five-millionths of an inch thick. The density of gold is 19.32 g/cm3. If gold costs $1768 per troy ounce (1 troy ounce = 31.10348 g), how much will it cost the architect to buy the necessary gold?
A copper refinery produces a copper ingot weighing 150 lb. If the copper is drawn into wire whose diameter is 7.50 mm, how many feet of copper can be obtained from the ingot? The density of copper is 8.94 g/cm3. (Assume that the wire is a cylinder whose volume 𝑉=𝜋𝑟2ℎ, where r is its radius and h is its height or length.)
Classify each of the following as a pure substance, a solution, or a heterogeneous mixture: b. a cup of coffee
