Write the formula for each of the following compounds.
(c) Tris(ethylenediamine)platinum(IV) sulfate
(d) Triamminetrithiocyanatorhodium(III)
 Verified step by step guidance
Verified step by step guidance

Write the formula for each of the following compounds.
(c) Tris(ethylenediamine)platinum(IV) sulfate
(d) Triamminetrithiocyanatorhodium(III)
Write the formula for each of the following compounds.
(a) Diamminesilver(I) nitrate
(b) Potassium diaquadioxalatocobaltate(III)
Write the formula for each of the following compounds.
(c) Hexacarbonylmolybdenum(0)
(d) Diamminebis(ethylenediamine)chromium(III) chloride
Tris(2-aminoethyl)amine, abbreviated tren, is the tetradentate ligand N(CH2CH2NH2)3. Using to represent each of the three NCH2CH2NH2 segments of the ligand, sketch all possible isomers of the octahedral complex [Co(tren)BrCl]+.
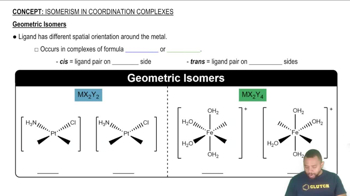
Consider the octahedral complex [Co(en)(dien)Cl]2+, where dien = H2NCH2CH2NHCH2CH2NH2, which can be abbreivated
(a) The dien (diethylenetriamine) ligand is a tridentate ligand. Explain what is meant by 'tridentate' and why dien can act as a tridentate ligand.
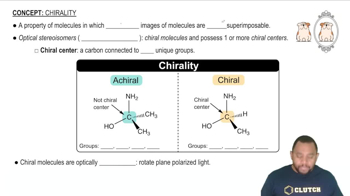
(b) Draw all possible stereoisomers of [Co(en)(dien)Cl]2+ (dien is a flexible ligand). Which stereoisomers are chiral, and which are achiral?