Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. For copper (Cu), the electron configuration is [Ar] 3d10 4s1, indicating that it has one unpaired electron in the 4s orbital. This unpaired electron is responsible for copper's paramagnetic properties, as unpaired electrons contribute to magnetic moments.
Recommended video:
Electron Configuration Example
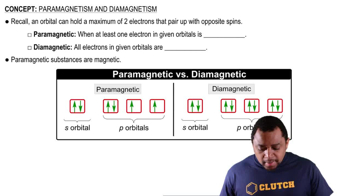
Paramagnetism
Paramagnetism is a form of magnetism that occurs in materials with unpaired electrons. These unpaired electrons create a net magnetic moment, allowing the material to be attracted to external magnetic fields. In the case of copper, the presence of the unpaired 4s electron makes it paramagnetic, while the absence of unpaired electrons in its 1+ ion (Cu+) results in a lack of magnetic properties.
Recommended video:
Paramagnetism and Diamagnetism
Ionization and Electron Removal
Ionization refers to the process of removing an electron from an atom or ion. When copper loses one electron to form Cu+, the electron is removed from the 4s orbital, resulting in the electron configuration [Ar] 3d10. This configuration has all electrons paired in the 3d subshell, leading to the conclusion that Cu+ is diamagnetic, as it lacks unpaired electrons.
Recommended video:
 Verified step by step guidance
Verified step by step guidance