Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
All textbooks McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 71
Problem 71
 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 71
Problem 71Chapter 8, Problem 71
Benzyne, C6H4, is a highly energetic and reactive molecule. What hybridization do you expect for the two triply bonded carbon atoms? What are the 'theoretical' values for the C¬C‚C bond angles? Why do you suppose benzyne is so reactive?
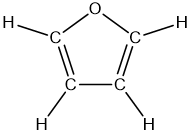
 Verified Solution
Verified SolutionVideo duration:
2m1407
views
Was this helpful?