Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Indicators
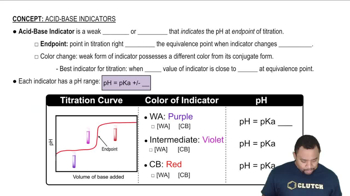
Acid-base indicators are substances that change color in response to changes in pH. They are typically weak acids or bases that exhibit different colors in their protonated and deprotonated forms. The choice of an indicator depends on the pH range over which it changes color, making it essential to select one that matches the expected pH change in a given reaction.
Recommended video:
pH Scale
The pH scale is a logarithmic scale that measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate basicity. Understanding the pH scale is crucial for selecting appropriate indicators, as each indicator has a specific pH range where it changes color.
Recommended video:
Color Change Range of Indicators
Each acid-base indicator has a specific color change range that corresponds to a particular pH interval. For example, bromocresol green changes from yellow to blue between pH 3.8 and 5.4, while phenol red changes from yellow to red between pH 6.8 and 8.4. To detect a pH change from 8 to 10, it is important to choose an indicator that changes color within this range, ensuring accurate detection of the pH transition.
Recommended video: