The Henry's law constant for CO2 in water at 25 °C is 3.1x10^-2 M atm-1. (a) What is the soubility of CO2 in water at this temperature if the soltuion is in contact with air at normal atmospheric pressure?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Henry's Law
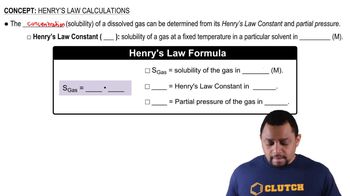
Solubility

Atmospheric Pressure

The following data were collected for the desturction of O3 by H (O3 + H → O2 + OH) at very low concentrations (b) Calculate the rate constant
The precipitation of Al(OH)3 (Ksp) = 1.3⨉10-33) is sometimes used ot purify water. (a) Estimate the pH at which precipitation of Al(OH)3 will begin if 5.0 lb of Al2(SO4)3 is added to 2000 gal of water
The valuable polymer polyurethane is made by a condensa- tion reaction of alcohols (ROH) with compounds that con- tain an isocyanate group (RNCO). Two reactions that can generate a urethane monomer are shown here: (i)
(ii)
(c) If you wanted to promote the formation of the isocyanate intermediate in each reaction, what could you do, using Le Châtelier's principle?
The pH of a particular raindrop is 5.6.
(a) Assuming the major species in the raindrop are H2CO3(aq), HCO3-(aq) and CO32- (aq), Calculate the concentrations of these species in the raindrop, assuming the total carbonate concentration is 1.0 * 10-5 M. The appropriate Ka values are given in Table 16.3.
(b) What experiments could you do to test the hypothesis that the rain also contains sulfur-containing species that contribute to its pH? Assume you have a large sample of rain to test.
