Here are the essential concepts you must grasp in order to answer the question correctly.
Rate Law
The rate law expresses the relationship between the rate of a chemical reaction and the concentration of its reactants. It is typically formulated as rate = k[A]^m[B]^n, where k is the rate constant, and m and n are the orders of the reaction with respect to each reactant. Understanding the rate law is essential for calculating the rate constant, especially in reactions involving low concentrations.
Recommended video:
Order of Reaction
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate law. It indicates how the rate of reaction is affected by the concentration of that reactant. For example, a first-order reaction depends linearly on the concentration of one reactant, while a second-order reaction depends on the square of the concentration. Identifying the order is crucial for determining the rate constant from experimental data.
Recommended video:
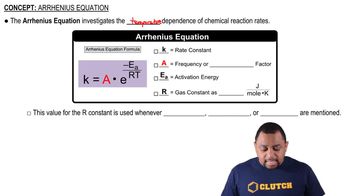
Arrhenius Equation
The Arrhenius equation relates the rate constant of a reaction to the temperature and activation energy. It is expressed as k = A * e^(-Ea/RT), where A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin. This equation helps in understanding how temperature influences the rate constant, which is particularly relevant when calculating k for reactions at varying temperatures.
Recommended video: