Here are the essential concepts you must grasp in order to answer the question correctly.
Equivalence Point in Titration
The equivalence point in a titration is the stage at which the amount of titrant added is stoichiometrically equivalent to the amount of substance in the sample. At this point, the reaction between the acid and base is complete, and the pH of the solution reflects the properties of the resulting salt and water. The pH at the equivalence point can provide insights into the strength of the acids involved.
Recommended video:
Equivalence Point in Titration
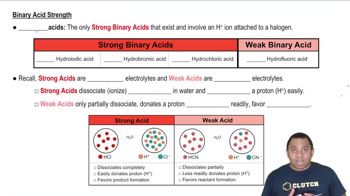
Strength of Acids
The strength of an acid is determined by its ability to donate protons (H+) in solution. Strong acids completely dissociate in water, resulting in a higher concentration of H+ ions, while weak acids only partially dissociate. The pH at the equivalence point can indicate the strength of the acid; a higher pH suggests a weaker acid, while a lower pH indicates a stronger acid.
Recommended video:
pH Scale and Acid-Base Behavior
The pH scale measures the acidity or basicity of a solution, ranging from 0 (very acidic) to 14 (very basic), with 7 being neutral. In the context of titrations, the pH at the equivalence point helps to classify the acid's strength. For the acids in this question, the pH values indicate that acid A, with the highest pH at equivalence, is the weakest, while acid C, with the lowest pH, is the strongest.
Recommended video: