Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Size and Representation
In molecular diagrams, the size of spheres often represents the relative size of atoms or ions. Larger spheres typically indicate atoms that are part of a solid structure, such as those in an electrode, while smaller spheres represent ions in solution. This visual distinction helps convey the difference in density and arrangement between solid and liquid phases.
Recommended video:
Electrode Function in Voltaic Cells
In a voltaic cell, electrodes serve as sites for oxidation and reduction reactions. The electrode is where electrons are transferred, and the larger spheres represent the solid metal atoms that facilitate this process. Understanding the role of electrodes is crucial for grasping how voltaic cells generate electrical energy through chemical reactions.
Recommended video:
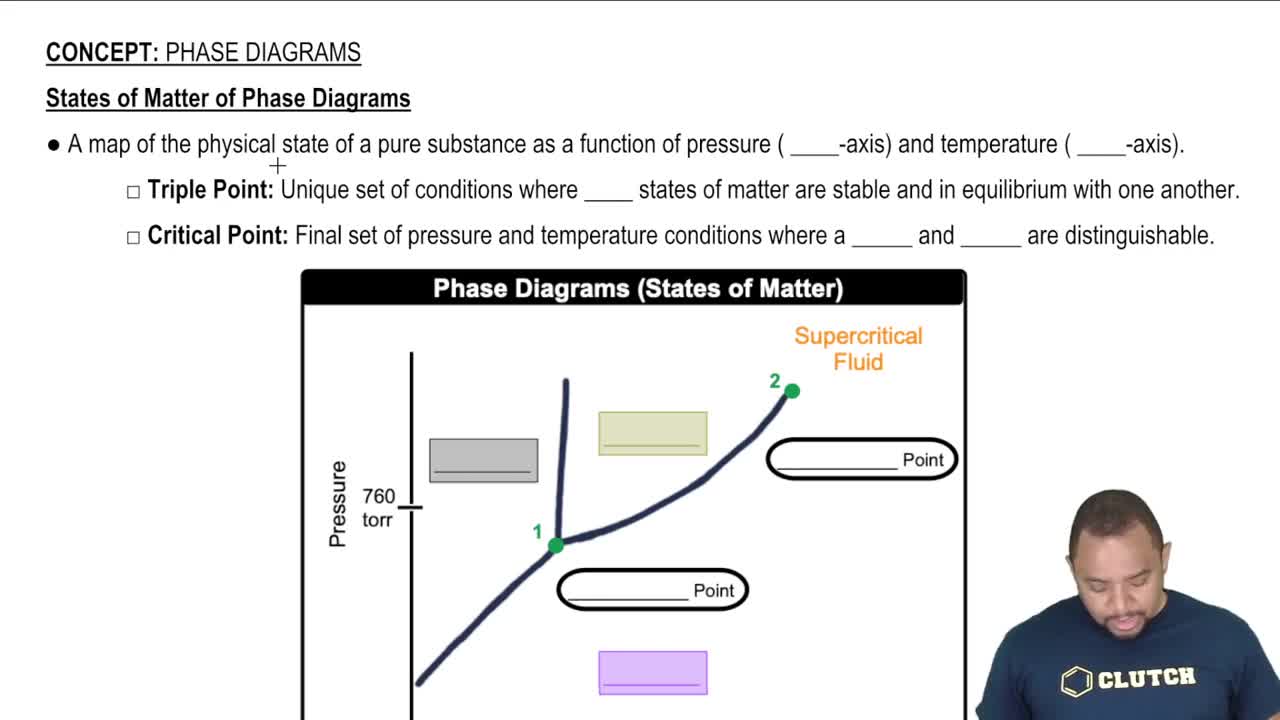
Phase Differences in Matter
The concept of phases in matter—solid, liquid, and gas—explains the differences in atomic arrangement and behavior. In solids, atoms are closely packed in a fixed structure, while in liquids, they are more dispersed and mobile. This distinction is essential for understanding why the electrode atoms appear larger and more organized compared to the smaller, more dynamic ions in solution.
Recommended video:
States of Matter of Phase Diagrams