Here are the essential concepts you must grasp in order to answer the question correctly.
Heat Transfer and Conservation of Energy
In thermodynamics, the principle of conservation of energy states that energy cannot be created or destroyed, only transferred. In this scenario, heat will flow from the warmer water to the cooler ice cubes until thermal equilibrium is reached. The total heat lost by the warm water will equal the total heat gained by the ice cubes, allowing us to calculate the final temperature.
Recommended video:
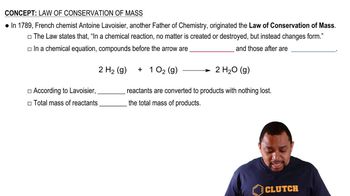
Law of Conservation of Mass
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. Different substances have different specific heat capacities, which affect how they respond to heat transfer. In this problem, the specific heat capacities of water and ice will be crucial for calculating the heat exchange and determining the final temperature.
Recommended video:
Phase Change and Latent Heat
When ice at 0 °C is introduced to warmer water, it may undergo a phase change from solid to liquid, which requires energy known as latent heat. This energy does not change the temperature of the ice but is essential for melting it. Understanding the latent heat of fusion for ice is necessary to account for the energy required to convert the ice cubes into water before reaching thermal equilibrium with the warmer water.
Recommended video:
 Verified step by step guidance
Verified step by step guidance