The structural formulas of the compounds n-butane and isobutane are shown below. (b) Determine the empirical formula of each.
Ch.2 - Atoms, Molecules, and Ions
Chapter 2, Problem 47a
What are the molecular and empirical formulas for each of the following compounds? Write the molecular formula for the following compound.


Verified Solution
Video duration:
45sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Formula
The molecular formula of a compound indicates the actual number of each type of atom present in a molecule. For example, dodecane has the molecular formula C12H26, which shows it contains 12 carbon atoms and 26 hydrogen atoms. This formula provides insight into the composition of the molecule but does not convey structural information.
Recommended video:
Guided course

Determining Molecular Formulas
Empirical Formula
The empirical formula represents the simplest whole-number ratio of the elements in a compound. For dodecane (C12H26), the empirical formula is C6H13, indicating that the ratio of carbon to hydrogen is 6:13. This formula is useful for understanding the basic composition of a compound without detailing the actual number of atoms.
Recommended video:
Guided course

Empirical vs Molecular Formula
Structural Formula
A structural formula provides a visual representation of the arrangement of atoms within a molecule, showing how atoms are bonded together. In the case of dodecane and methane, the structural formulas illustrate the linear chain of carbon atoms in dodecane and the tetrahedral arrangement of hydrogen atoms around a single carbon atom in methane. This information is crucial for understanding the chemical behavior and properties of the compounds.
Recommended video:
Guided course
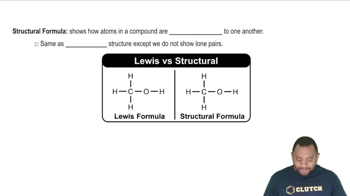
Structural Formula
Related Practice
Textbook Question
815
views
Textbook Question
The structural formulas of the compounds n-butane and isobutane are shown below. (c) Which formulas—empirical, molecular, or structural—allow you determine these are different compounds?
604
views
Textbook Question
Ball-and-stick representations of benzene, a colorless liquid often used in organic chemistry reactions, and acetylene, a gas used as a fuel for high-temperature welding, are shown below. (a) Determine the molecular formula of each.
427
views
Textbook Question
Two substances have the same molecular and empirical formulas. Does this mean that they must be the same compound?
922
views
Textbook Question
Write the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6
776
views
Textbook Question
Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10
1017
views
